Quartz is more than a crystal; it is a crucial mineral in various industries. As Dr. Emily Chen, an expert in Quartz Chemical properties, once stated, "Understanding quartz is key to unlocking its full potential in technology." This underscores the importance of grasping the nuances of Quartz Chemical properties.
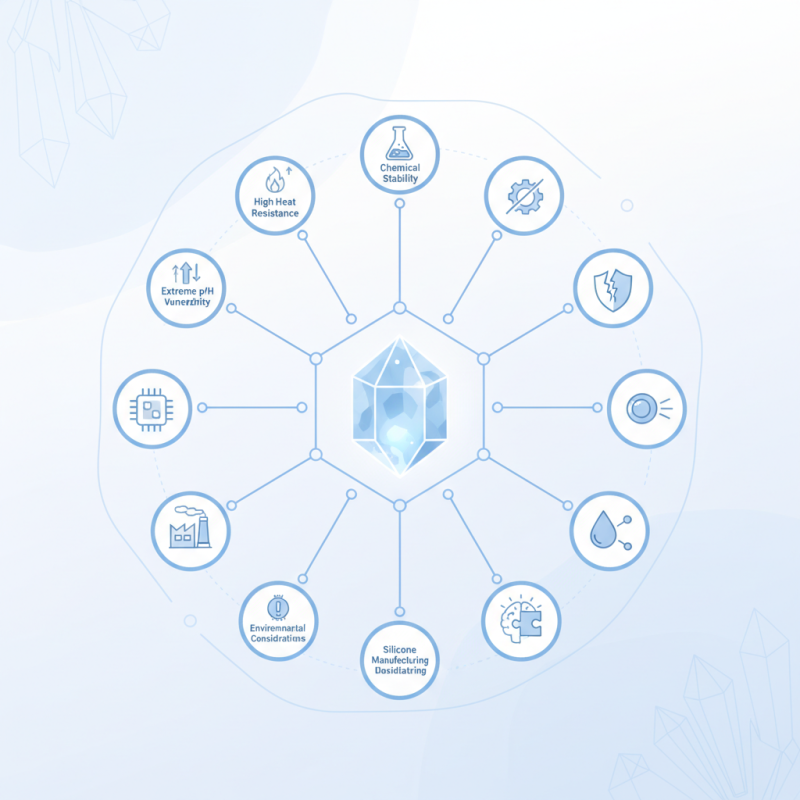
To effectively work with quartz, one must delve into its unique characteristics. Quartz has a high resistance to heat and chemical reactions. However, its behavior changes under specific conditions, revealing both strengths and weaknesses. For example, while quartz is durable, it can be altered by extreme pH levels.
In applications, quartz serves in electronics, optics, and even in manufacturing silicone. Yet, one must always consider the implications of using quartz in different environments. Many industries face challenges that stem from a lack of understanding of quartz's chemical interactions. Hence, a thorough understanding is essential for harnessing its true capabilities.
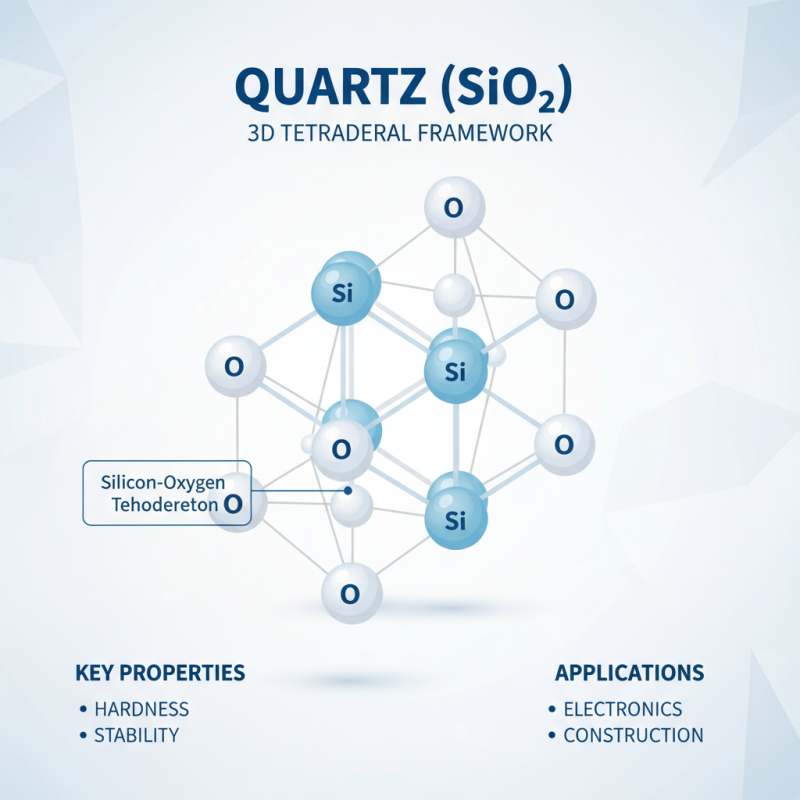
Quartz, primarily composed of silicon dioxide (SiO₂), has a fascinating chemical structure. This structure features a three-dimensional framework of silicon and oxygen atoms. Each silicon atom bonds with four oxygen atoms, creating a tetrahedral shape. This arrangement contributes to quartz's hardness and stability. Understanding this structure is crucial for various applications, from electronics to construction.
One essential tip for grasping quartz's properties is to recognize its crystalline nature. This property affects its physical characteristics and behavior in different environments. Another key point is quartz's thermal stability. It withstands high temperatures, making it suitable for many industrial processes. Pay attention to its transparency and refraction qualities as well; they play a pivotal role in optical applications.
Consider that while quartz is widely used, it's essential to reflect on its limitations. For example, its brittleness can be a drawback in some applications. This acknowledgment can lead to research on improving materials. Overall, understanding quartz's chemical structure provides valuable insights into its myriad uses and potential for innovation.
Quartz, a common mineral in the Earth's crust, boasts unique chemical properties. It primarily consists of silicon dioxide (SiO2) and forms a sturdy crystalline structure. This makes quartz resistant to many chemical reactions. However, its stability can be influenced by the surrounding environment. For example, elevated temperatures and pressures can alter its properties, leading to various forms of quartz.
The implications of quartz's chemical properties are vast. Its inertness allows it to be used in applications like glass production and electronics. Despite its durability, quartz can suffer from weathering. Acid rain can slowly dissolve its surface, affecting its integrity. In some studies, it was noted that prolonged exposure could lead to significant changes in its structure. Understanding these properties is essential for various industries, from construction to technology. The versatility and shortcomings of quartz make it crucial for innovation and application.
Quartz is a remarkable mineral with numerous applications across various industries. Its chemical properties, such as hardness and resistance to weathering, make it ideal for many uses. In construction, quartz is often used in countertops and tiles due to its durability. Its vibrant colors and patterns can create stunning aesthetics in homes and offices.
In technology, quartz plays a crucial role in electronics. Quartz crystals are fundamental in timekeeping devices like watches. These crystals help maintain accurate time because they vibrate at a stable frequency. Furthermore, quartz is utilized in the manufacturing of semiconductors, essential for modern electronic devices. This use highlights the ongoing demand for quartz in tech innovation.
While quartz has many benefits, there are challenges. For instance, sourcing high-quality quartz can be difficult. Environmental concerns also arise in mining operations, prompting questions about sustainability. Reflecting on these issues is vital as industries strive for more responsible practices. Recognizing both the advantages and the downsides of quartz usage can lead to better decision-making in future applications.
| Chemical Property | Description | Application in Industry | Application in Technology |
|---|---|---|---|
| Hardness | Quartz has a hardness of 7 on the Mohs scale, making it resistant to scratching. | Used in the production of abrasives and cutting tools. | Utilized in making watch faces and smartphone screens. |
| Chemical Stability | Quartz is chemically inert and does not react easily with acids and bases. | Ideal for laboratory equipment and chemical storage. | Used in semiconductor manufacturing due to low reactivity. |
| Thermal Resistance | Can withstand high temperatures without losing its properties. | Used in furnace linings and other high-temperature applications. | Cooling systems in electronic devices benefit from thermal resistance. |
| Transparency | Quartz is transparent to visible light, with excellent optical clarity. | Used in lenses and other optical devices. | Utilized in fiber optics for communication technologies. |
| Piezoelectric Properties | Quartz generates electrical charge when mechanically stressed. | Used in sensors, actuators, and pressure gauges. | Common in clocks and watches for precise timekeeping. |
| Color Varieties | Comes in various colors due to impurities (e.g., amethyst, citrine). | Used in jewelry manufacturing and decorative items. | Enhances aesthetic appeal in technology products. |
| Density | Relative density of quartz is about 2.65 g/cm³. | Important for applications requiring specific weight considerations. | Used in specialized equipment where weight balance is critical. |
| Electrical Conductivity | Low electrical conductivity, making it a good insulator. | Used in insulation materials for electrical components. | Key in insulation for sensitive electronic devices. |
| Durability | Highly durable and resistant to mechanical impact. | Used in construction materials like countertops and tiles. | Critical for devices needing robust casings. |
| Environmental Resistance | Quartz is resistant to weathering and environmental conditions. | Used in outdoor applications and building facades. | Improves longevity of technology exposed to harsh environments. |
The extraction of quartz can have significant environmental impacts. Mining activities disrupt ecosystems. The land becomes scarred, and habitats are destroyed. This disruption can lead to a loss of biodiversity. Water sources may also be contaminated with sediment and chemicals. Such damage can take years, if not decades, to restore.
Additionally, quartz processing generates waste. This waste can include harmful byproducts that leach into the soil. Dust and particulate matter from mining operations can affect air quality. Local communities often bear the brunt of these effects. They might face health issues and economic challenges due to environmental degradation.
While quartz is valuable in various industries, we must reflect on the costs. The benefits may be tempting, but the extraction process can be harmful. Finding a balance is crucial. Sustainable practices in quartz extraction and use are essential. Awareness can lead to more responsible choices in sourcing this important mineral.
Quartz is a mineral with versatile applications. Recent research trends highlight innovative uses in technology and industry. A report by MarketsandMarkets predicts a 4.6% CAGR for quartz market size from 2021 to 2026. This growth is fueled by its increasing usage in electronics and renewable energy technologies.
As researchers explore quartz's chemical properties, new applications emerge. For instance, the integration of quartz in semiconductor manufacturing is gaining traction. This adaptation could revolutionize the electronics sector. Understanding the chemical stability of quartz allows for enhanced performance in various uses.
Tip: Always consider quartz purity and the presence of trace elements. This can significantly affect its performance in specific applications.
Moreover, sustainable sourcing of quartz is becoming crucial. With environmental concerns on the rise, researchers are focusing on eco-friendly extraction techniques. The aim is to reduce the carbon footprint of quartz production. However, quantifying this impact remains a challenge.
Tip: Stay informed about advancements in eco-friendly quartz mining practices. They are vital for future innovations. Engaging in these discussions helps frame a responsible approach to quartz usage.